Abstract
Background: Thrombocytopenia (TCP) is common in patients with chronic liver disease (CLD). These patients may require platelet transfusions before routine procedures associated with a bleeding risk, thereby exposing them to the risks of transfusion reactions, infections and platelet alloimmunization, while complicating the performance of the procedure and impacting platelet supplies. Avatrombopag (AVA) is an oral, 2ndgeneration, small molecule thrombopoietin receptor agonist being developed to provide a measured increase in platelet counts (PC) as an alternative to platelet transfusions. Two randomized, double-blind, placebo (PBO)-controlled Phase 3 trials evaluated the potential for AVA to increase PC and reduce the need for platelet transfusions in patients with CLD undergoing scheduled procedures.
Methods: ADAPT1 (NCT01972529) and ADAPT2 (NCT01976104) enrolled adults with CLD and TCP (mean Baseline PC <50x109/L), scheduled to undergo a procedure. Cohorts were based on Baseline PC (Cohort 1 - <40x109/L, or Cohort 2 - 40 to <50x109/L), and patients randomized 2:1 to receive once-daily oral AVA (Cohort 1- 60 mg; Cohort 2 - 40 mg) or PBO for 5 days with the procedure scheduled 5 to 8 days after the last dose (Day 10 to 13).
The primary efficacy endpoint was the proportion of patients not requiring platelet transfusion or any bleeding rescue procedure up to 7 days post-procedure. Secondary endpoints assessed the proportion of patients achieving the target PC of ≥50x109/L by Procedure Day, change in PC from Baseline to Procedure Day, and safety.
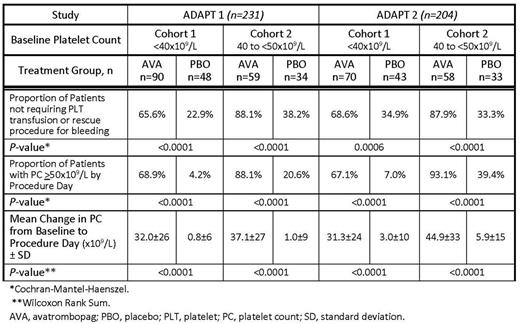
Results: Together, these studies randomized 435 patients. ADAPT1included 231 patients (median age 57 y; 68% male; Baseline median PC 38x109/L; CLD etiology: alcohol 14%, viral hepatitis 62%, other 23%). In Cohort 1, 90 patients were randomized to AVA and 48 to PBO and in Cohort 2, 59 were randomized to AVA and 34 to PBO. ADAPT2randomized 204 patients (median age 59 y; 62% male; Baseline median PC 39x109/L; CLD etiology: alcohol 15%, viral hepatitis 53%, other 33%). In Cohort 1, there were 70 patients who received AVA and 43 PBO, and in Cohort 2, 58 were randomized to AVA and 33 to PBO.
Both studies met their primary efficacy endpoint, demonstrating the superiority of AVA to PBO in increasing the proportion of patients not requiring a platelet transfusion or rescue procedure for bleeding with treatment differences of ADAPT1: Cohort 1 - 42.6% (P<0.001), Cohort 2 - 49.9%, (P<0.0001); ADAPT2: Cohort 1-33.7% (P=0.0006), Cohort 2 - 54.6% (P <0.0001). In addition, a significantly higher proportion of AVA-treated patients successfully achieved the target PC of ≥50x109/L by Procedure Day compared with PBO in both cohorts in ADAPT1 and ADAPT2 (Table). Further, the magnitude of the change in mean PC from Baseline to Procedure Day was significantly larger in AVA-treated compared with PBO-treated patients in both ADAPT1 and ADAPT2in both cohorts (Table). Only 3 AVA-treated subjects achieved PC ≥200x109/L; all were asymptomatic.
In both Phase 3 studies, the mean PC in AVA-treated patients started increasing on Day 4 and peaked at ~2 times Baseline PC on Procedure Day (Day 10 to 13); by 7 days post-procedure, mean PC had decreased in both AVA groups, returning to Baseline by Day 35.
The most common treatment-emergent adverse events (TEAE) were pyrexia, abdominal pain, nausea, and headache. TEAEs were similar for AVA and PBO arms in both studies. Most TEAEs were mild to moderate in severity; one thrombotic TEAE (partial right portal vein thrombosis) occurred with 40 mg AVA in Cohort 2 in ADAPT2 on Day 7 post-procedure, 14 days after the last AVA dose.
Conclusions: Both Phase 3 studies demonstrated the superiority of AVA to PBO in increasing the proportion of patients not requiring a platelet transfusion or any rescue procedure for bleeding. AVA given orally over 5 days resulted in a significant increase in patients achieving the target PC of ≥50x109/L by Procedure Day compared to PBO. The mean change in PC from Baseline to Procedure Day was significantly greater for AVA-treated patients than those who received PBO. In both studies, AVA-treated patients' PC peaked at ~2 times Baseline on Procedure Day, and returned to Baseline by Day 35. AVA was well tolerated, with a safety profile similar to PBO. An agent that provides a measured increase in PC and reduces the need for platelet transfusions may be advantageous for patients with CLD and TCP undergoing invasive procedures to importantly reduce their risks.
Terrault: Merck: Research Funding; AbbVie: Research Funding; Gilead: Research Funding; BMS: Research Funding; Allergan: Research Funding; Eisai: Research Funding. Kuter: Pfizer: Consultancy; Genzyme: Consultancy; Merck: Consultancy; Shire: Consultancy, Research Funding; Protalex: Consultancy, Research Funding; 3SBIO: Consultancy; ONO: Consultancy; BMS: Consultancy, Research Funding; Rigel: Consultancy, Research Funding; Alexion: Consultancy, Research Funding; Dova: Consultancy, Membership on an entity's Board of Directors or advisory committees; Argenx: Consultancy; Incyte: Consultancy, Research Funding; Fujifilm: Consultancy; Zafgen: Consultancy; Amgen: Consultancy; Syntimmune: Consultancy, Research Funding; Novartis: Consultancy. Bibbiani: Eisai, Inc.: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal